Easyone Frontline Spirometer Manual
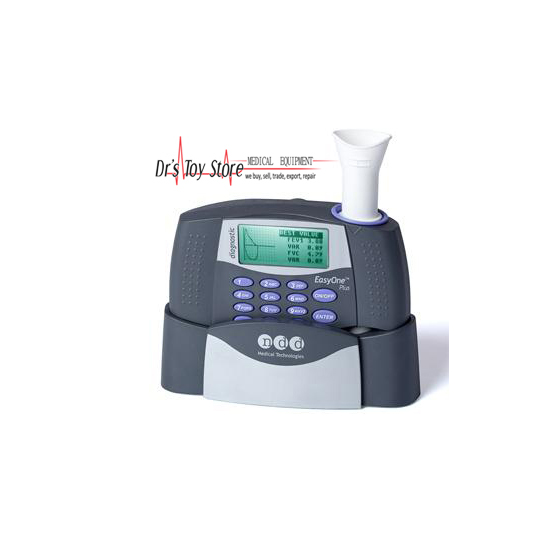
Page/Link:Page URL:HTML link:The Free Library. Retrieved Oct 06 2019 fromIn this issue of Focus we report our evaluation of the EasyOneDiagnostic Spirometer by ndd Medical Technologies.The EasyOne Spirometer is small, light weight and battery powered.It is designed for bedside, outpatient, including industrial screeningclinics, Emergency room or office use. With its graphic and numericdisplays, its wide range of testing parameters and its comprehensivereport format, this diagnostic device has the potential to documentpulmonary function status in many different settings and conditions.Additionally, we found that the spirometer has the capacity to store anamazing amount of test data.ILLUSTRATION OMITTEDSize - the EasyOne's footprint is a mere 3.3' X6.2' X 1.7' (8.25 X 15.5 X 9.25 cm).Weight - 9.0 oz (252 grams).Power Requirements - 2 AA batteries (400 tests).Resistance. ILLUSTRATION OMITTEDOutputOn board-is a 64 X 160 graphic display screen with both graphicand alpha numeric capability. Out board-data can be directed to a PC orprinter through standard USB 'A' and 'B' or DB25connectors in the docking module.
A PC data base and patient instructionprogram are among several available software applications for PC usersincluded along with the provided Easy Ware CD.Test ModesTwo test modes are available. The first mode (the Diagnostic mode)meets ERS (European Respiratory Society) and ATS (American ThoracicSociety) standards and is also configurable to NIOSH/OSHA and disabilityreporting requirements.
The second mode (called the Frontline mode),offers a simpler, less rigorous protocol suitable for screening andfirst line labs. The diagnostic mode also offers a selection of normalvalue standards as opposed to the fixed NHANESIII normal values used inthe Frontline mode. Additionally the Diagnostic mode has enhanced datastorage and quality control requirements and a user customized reportform.
Ethnic correction factors are available for Caucasian,Afro-American, Hispanic, Asian and Other patients with the Caucasianvalues being the default criteria and the remaining ethnic correctionmade to those values. These corrections are referenced to the ATS'Lung Function Testing: Selection of Reference Values andInterpretive Strategies' document (Am Rev of Respir Dis 1991; 144;1202-1218).ManualsThe manual for this instrument is thorough and easy to follow. Goodclear photographs illustrate many set-up and use steps. Cautions andhelpful hints are boxed to highlight their messages. The manual alsoincludes a simple algorithm for basic interpretation.
Normal valuesources are referenced for both adults (8) and children (4) and generalreferences are cited. Basic patient instructions, QC information andtest interventions are also provided along with detailed test by testinstructions.
Easyone Spirometer Software
An interesting 'Quality Grade' chart presents ascheme to rank the 'quality' of the test session from A, (bestquality) to progressively lesser quality B, C, D, or F grades with D andF scores being unacceptable. These can be added to the report or may besuppressed. We thought this a nifty feature as well. Finally, there isample white space in the manual for notes or comments.The EasyOne Diagnostic spirometer is a compact device that does notrequire the use of a cable connection to a pneumotach.
User manual; Find Similar Products by Tag. Click the button below to add the ndd EasyOne Plus Diagnostic Spirometry System I to your wish list. Related Products. Ndd EasyOne Plus Diagnostic Spirometry System II $1,000.00. Ndd EasyOne Plus Frontline Spirometry System $500.00. You Recently Viewed. Ndd EasyOne Plus Diagnostic Spirometry.
The disposableflow tube fits directly into the device in such a way that it can not beinserted improperly without using excess force (another nice feature).In addition, there are places on each side for the patient to grip theunit between two hands and hold it up to the mouth for testing. Of course, the most unique feature about the EasyOne is its use ofUltrasonic Flow Measurement technology. Back in 1986 Dr. Christian Buessdeveloped an ultrasound flow sensor at the Institute for Electronics atthe Swiss Federal Institute of Technology. Flows sensors of this typewere also used in one of the shuttle missions and in the Russian MIRStation. In 1992 a cooperation between ndd and reseacher Prof. KarlHarnoncourt was established with the goal of developing an ultrasoundflow sensor for clinical routine applications.
This cooperation soonlead to the foundation of ndd Medizintechnik AG and to the developmentof the EasyOne spirometer.Ultrasonic flow measurement eliminates problems associated withtraditional methods of flow measurement and helps make the EasyOnespirometer fast and accurate. There are no moving parts, no codes toenter, no screens to catch sputum, and no disposables to calibrate andsince ultrasonic flow measurement is independent of gas composition,pressure, temperature, and humidity; it eliminates errors due to thesevariables.Transducers located on either side of the spirette cavity emit andreceive sound in alternating directions. When gas flow is present in thetube, a pulse traveling against the flow (traveling upstream) is sloweddown and takes a longer time to reach the opposite transducer.Conversely, a pulse traveling with the flow (traveling downstream) issped up and takes a shorter time to reach the opposite transducer.The transit time of the sound pulses is precisely measured with adigital dock. The gas flow through the spirette is then calculated fromthe upstream and downstream transit times.
This calculation isindependent of gas composition, pressure, temperature, and humidity. Thedisposable spirette then, acts only as a hygienic shield and istransparent to the ultrasonic pulses traveling between the measurementtransducers. Since the disposable spirette has no sensor elements, itdoes not perform a measurement function and does not requirecalibration.Test Conditions and ResultsFor calibration we used a standard 3 liter syringe and theappropriate calibration volume and procedures designated in the EasyOnemanual. Mapilab nntp for outlook cracker. We used the 3 liter syringe to create several conditions, highflow, low flow, 3L and 1L volumes. Differences in volume variedaccording to flow, as is often seen with spirometers.
Easyone Frontline Spirometer Manual
At 3 L low flowmeasurements ranged from 3.02 to 3.16 L. High flow measurements rangedfrom 3.26 to 3.32 L.
At 1 L low flow measurements ranged from 1.00 to1.24 L and high flow measurements ranged from 1.00 to 1.28 L. Underreading volumes was not a problem during simulated test conditions.After successfully calibrating the unit, we performed measurementson individual subjects. Obstructive and restrictive conditions weretested, and all satisfactorily compared to laboratory based pulmonaryfunction equipment. Volume measurements under these circumstances wereslightly, but insignificantly, higher than those obtained with atraditional bench based spirometer when no airflow obstruction waspresent.
There were no situations where the spirometer under reportedthe volume measurements although we noticed that in cases of severeupper airway obstruction the patient's severe state of obstructionterminated testing prematurely. This, again, is a problem seen in manytypes of spirometer, portable or lab-based. We also felt that the flowtube itself was a little too soft and could be easily collapsed(resulting in obstructed airflow at high flow rates) if there was atendency to bite down on the tube by the patient. This situation, ofcourse, can easily be remedied by using a harder plastic tube at themouthpiece and by instructing the patient not to bite down on the tubeor mouthpiece. By the way, the mouthpiece that can be ordered from nddwas molded in a unique way that we felt was more comfortable than thestandard spirometer mouthpiece.Data entry for patient testing is intuitive enough. The 14 keykeyboard is modern and compact, definitely easy for any therapist tomaster. Naturally, it does not have the functionality of a fullkeyboard, which would be impractical considering the compact size of theunit, but the EasyWare software provided interfaces the spirometer baseunit to a computer.
As a result, data can then be easily entered via atraditional sized keyboard and uploaded to the spirometer. Since thedevice is disconnected from the base for testing, the patientinformation is displayed by using the recall patient function on thespirometer menu. After completing tests, the spirometer is reconnectedto the base and the data downloaded from the spirometer into thedatabase stored on the PC or lap-top.Viewing the data is also straight forward. Selecting the desiredtest from a spreadsheet window opens the display window. Flow-volumeloops and volume-time graphs are displayed in standard fashion alongwith the spirometry data in tabular form.
Easyone Pro Lab Manual
The graphic elements can bedisplayed as either a single best test or as the best three trials withthe best test of those displayed in bold. A table displays the numericaldata of the three best tests.
The best of those tests is also indicatedwith a highlight over the column of data. Print displays are formattedto conform to ATS criteria and can be previewed with the EasyWaresoftware.
The diagnostic criterion used for interpreting the data wasmost helpful, and we agreed with the interpretation of both the severityand type of lung process, obstructive versus restrictive in all cases.ConclusionThe EasyOne diagnostic spirometer and EasyWare software combinationoffers acceptable spirometry results for office, hospital or industrialuse. It is easily portable, but in combination with a traditionalcomputer offers more options than many stand alone portable units thatdo not have a PC-software option. The formats are acceptable forpermanent medical records and meet the criteria for ATS/ETS andDisability reports.

Operating the device is easily learned and applied,and offers a good value for use in most clinical situations. Werecommend your looking into ndd's EasyOne the next time you'rein the market for a portable spirometer. Ndd can be reached by calling877-904-0090. Their website is located at www.nddmed.comILLUSTRATION OMITTEDPaul Mathews PhD, RRT & Michael Czervinske, BS, RRT.